Blood Collection
At Equilibrium Diagnostics, we make the blood collection process safe, simple, and comfortable. Whether you’re testing for wellness, diagnostics, or specialized panels, our certified phlebotomists ensure a smooth experience from start to finish.
Blood Collections Guidelines
Proper specimen collection is important in providing timely and accurate patient care. Improper collection and/or recollection of specimens may delay important procedures or medication changes. In addition, suboptimal specimens may provide erroneous results. Equilibrium Diagnostics suggests the following:
1. Specimen Collection
Specimens should be collected in order per the chart below to prevent potential contamination from one tube to the next.
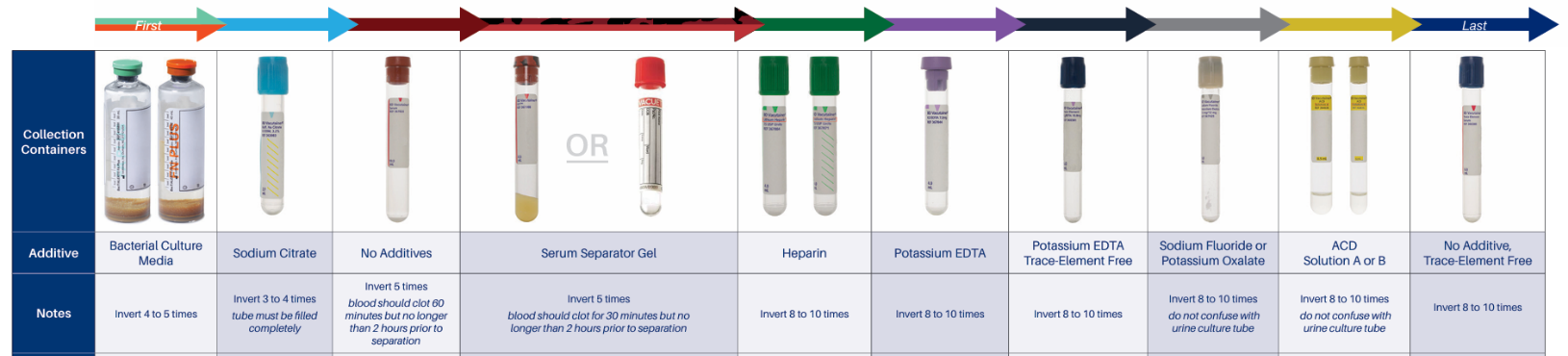
⦁ Specimen rejections may occur due to interfering substances if a lavender top tube is collected before a serum tube. EDTA from the lavender top could contaminate the serum specimen, causing an erroneously elevated potassium level and decreased calcium level.
⦁ Inverting the tubes the number of times indicated on the chart will distribute the anticoagulant or other additive throughout the specimen and prevent clotting for whole blood or promote clotting in serum tubes.
⦁ If there is an error in collection, do not pour from one tube to another. For example, if a lavender tube is not collected, you cannot pour blood collected in a red top into a lavender top, regardless of the time of collection. This would cause clotting to occur in the lavender top.
2. Labeling
First name, last name and date of birth. In the presence of the patient, label each specimen with the patient’s first name, last name and date of birth. Time and date of collection. Adding time and date of collection helps to evaluate each specimen for stability and acceptability. Barcode. If a barcode label is added to the tube, make sure it does not obscure patient information.
3. Processing
Let all serum separator tubes (ST) clot upright in a tube rack, for at least 30 minutes. Let plain red top (RT) tubes clot upright for at least 60 minutes. This prevents the blood clot from sticking to the top of the tube while clotting. ST and RT tubes should be centrifuged within 2 hours of collection to preserve analyte stability. The gel barrier in a ST tube must be well formed to ensure red cells do not come in contact with the serum, as prolonged contact between the serum and red cells can falsely increase potassium and decrease glucose. If there are red cells in the serum, the serum should be pipetted into a transfer tube, centrifuged again and separated into another transport tube.
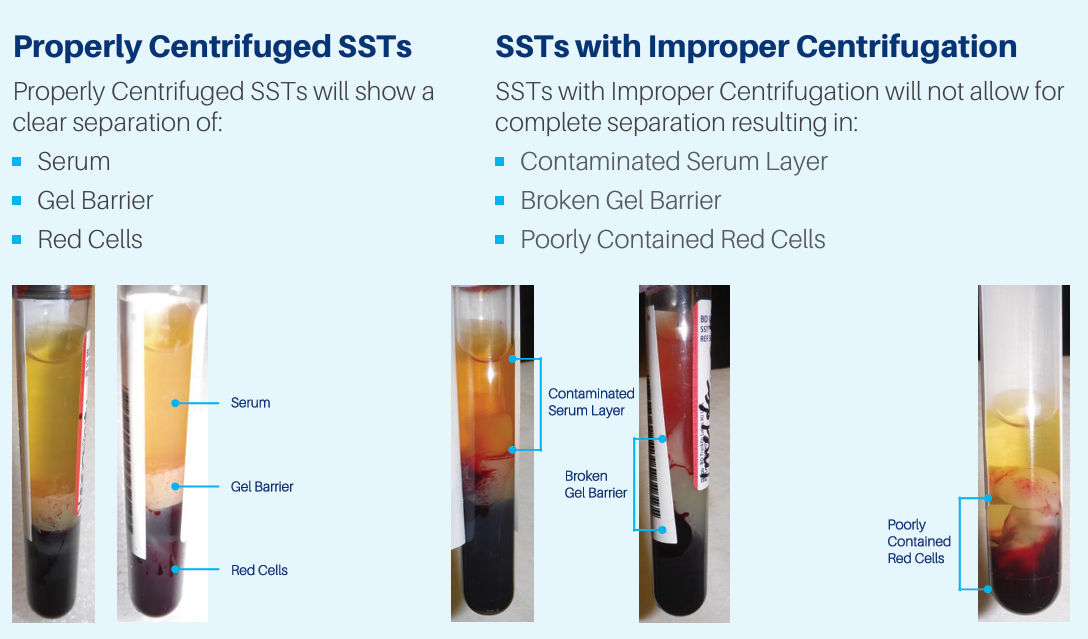
At times, serum or plasma may need to be separated from cellular material. If that is the case, use a plastic pipette to gently remove the serum or plasma without touching the red cell layer. Express plasma or serum into a plastic transport tube. Be sure to label the tube with the specimen type, e.g., serum from a plain red. If you have a test or specimen that requires freezing, do not freeze the specimen in the original collection tube. Process the specimen as required, transfer the correct specimen to a transport tube, label with the patient identifiers, time and date of collection, specimen type, and freeze upright. Place a copy of the test request in the transport bag with the frozen specimen.
Stool Collections Guidelines
| Collection Container | Test Name | Storage/Transport Temp | Specimen Requirements | Deliver to Laboratory Within |
|---|---|---|---|---|
| Stool culture | Stool culture | Refrigerated (2-8 °C) | Fresh stool in Cary-Blair transport media | 24hrs after collection |
| Vibrio culture | Vibrio culture | Refrigerated (2-8 °C) | Fresh stool in Cary-Blair transport media | 24hrs after collection |
| Yersinia culture | Yersinia culture | Refrigerated (2-8 °C) | Fresh stool in Cary-Blair transport media | 24hrs after collection |
| E. coli 0157:H7 culture with shigatoxin | E. coli 0157:H7 culture with shigatoxin | Refrigerated (2-8 °C) | Fresh stool in Cary-Blair transport media | 24hrs after collection |
| Shigatoxin | Shigatoxin | Refrigerated (2-8 °C) | Fresh stool in Cary-Blair transport media | 24hrs after collection |
| C. difficile GDH reflex toxin/PCR | C. difficile GDH reflex toxin/PCR | Refrigerated (2-8 °C) | Fresh stool in stool collection container. If test cannot be performed within 72hrs., freeze sample at -20 °C | 24hrs after collection |
| C. difficille toxin by PCR | C. difficille toxin by PCR | Refrigerated (2-8 °C) | Fresh diarrheal stool in stool collection container | 24hrs after collection |
Extended Stool Test Requirements
| Test Name | Storage/Transport Temp | Specimen Requirements | Deliver to Laboratory Within |
|---|---|---|---|
| H. pylori antigen | Refrigerated (2-8 °C) | Fresh stool in a Stool Collection Container. Avoid antibiotics, PPIs, or bismuth 14 days prior. | Refrigerated: 2 days, Frozen: 3 days after collection |
| Osmolality | Frozen | Liquid stool only. Freeze immediately. Do not add saline or water. | 24hrs after collection |
| Porphyrins | Frozen, wrap in foil | Formed stool. Wrap container in foil. Freeze immediately. | 24hrs after collection |
| Electrolytes (Sodium, Potassium, Chloride) | Frozen (≤ -20 °C) | Stool in collection container | 24hrs after collection |
| Magnesium | Frozen (≤ -20 °C) | Stool in collection container | 24hrs after collection |
| Pancreatic Elastase | Frozen (≤ -20 °C) | Stool in collection container | 24hrs after collection |
| Sodium | Refrigerated (2-8 °C) | Liquid stool in stool collection container | 24hrs after collection |
| Parasite Antigen Profile | Refrigerated (2-8 °C) or Frozen (≤ -20 °C) | Fresh stool in collection container | Refrigerated: 12 hrs, Frozen: 3 days after collection |
| Fecal Calprotectin | Refrigerated (2-8 °C) | Fresh stool in collection container | 24hrs after collection |
| pH | Refrigerated (2-8 °C) | Fresh stool in collection container | 24hrs after collection |
| Fecal Fat, Qualitative | Frozen (≤ -20 °C) | Freeze stool within 1 hour of collection | 24hrs after collection |
| Reducing Substance | Frozen | Stool in collection container, freeze | 24hrs after collection |
| Occult blood, fecal, guaiac (screen 1-3) | Room Temp (15–25 °C) | Stool in collection container | — |
| Fecal Fat, Quantitative | Frozen (≤ -20 °C) | Pre-weighed container from lab. Collect 24-72 hrs. Keep refrigerated during collection. Diet: 50–150g fat/day for 3 days prior. | 24hrs after collection |
| Ova and Parasites with Trichrome Stain | Room Temp (15–25 °C) | Stool in 10% Formalin and PVA preservative kit (1-3 sets) | 3 days after collection |
| Fecal Leukocytes | Room Temp (15–25 °C) | Stool or colon washings in 10% Formalin and PVA kit | 3 days after collection |
| Giardia Antigen | Room Temp (15–25 °C) | Stool in 10% Formalin Vial | 3 days after collection |
| Parasite Stain | Room Temp (15–25 °C) | Use collection spoon to fill vial to arrow line. Avoid urine/water. Max 3 specimens in 7–10 days. | 3 days after collection |
| Gastrointestinal Panel | Refrigerated (2-8 °C) | Rectal swab or orange top stool | 24hrs after collection |
| Occult Blood, Fecal, Immunoassay (MC SCR) | Room Temp (15–25 °C) | Stool in Polymedco Green Cap Collection Paddle | 24hrs after collection |